A group of scientists from the Max Planck Research Group at the Malopolska Centre of Biotechnology (MCB) of the Jagiellonian University in collaboration with the University of Queensland from Brisbane, Australia has shed light on the genetic and molecular cause of severe neurological diseases in humans. The results of their studies have recently been published in Nature Communications.
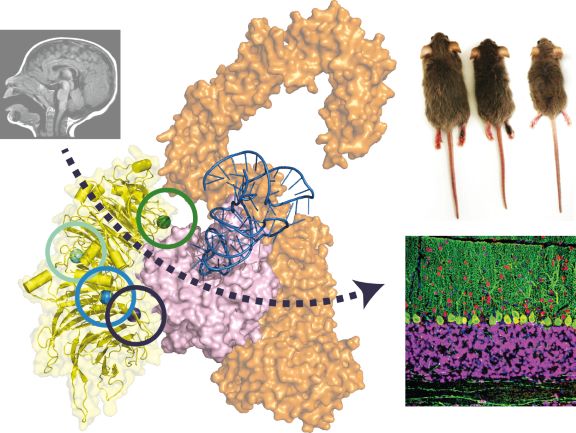
For the last 3 years they were working intensively on examining an association between mutations in a gene coding one of the subunits of the Elongator complex and the development of microcephaly, intellectual disability (ID) and autism spectrum disorder (ASD). “Elongator is a multi-subunit protein complex that introduces chemical modifications to tRNA molecules playing a central role in translation – a process that regulates the production of all proteins in every living organism.”– explains Tomasz Gawda, a Master student at Max Planck Research Group and a second author of the publication. “Mutations in any of the Elongator subunits are associated with a variety of human diseases, but the underlying mechanisms have not been understood until now.” – adds Dr. Monika Gaik.
Their highly multidisciplinary approach combined clinical data obtained from patients in Australia, Denmark, Germany, the United States of America and France with in vivo experiments on murine disease models, including detailed brain scans and unique behavioral tests. The biomedical research was complemented by a team of 7 scientist from Poland, who added advanced biochemical studies using purified human proteins, which showed the direct impact of each patient-derived mutations on the molecular level.
In summary, the study reveals that patient-derived mutations in the Elongator protein 2 (Elp2) have a profound impact on neurodevelopment through direct inhibition of Elongator’s enzymatic activity. “It is the first time that we are able to purify this large human protein complex and directly study the detrimental effects caused by mutations in the lab. Our biochemical and biophysical analyses of the mutations found in ID and ASD directly confirmed that the protein is responsible for the observed clinical phenotypes.” – says Dr. Sebastian Glatt, the co-corresponding author of the paper, who together with Prof. Brandon J. Wainwright from the IMB in Brisbane initiated and coordinated the research project.
The presented data provides a novel detailed picture of the highly complex etiology of intellectual disabilities and autism as well as the fundamental role of the Elongator complex in health and disease. The results will allow scientists from all over the world to explore new diagnostic markers and potential intervention therapies for these severe neurodevelopmental disorders.